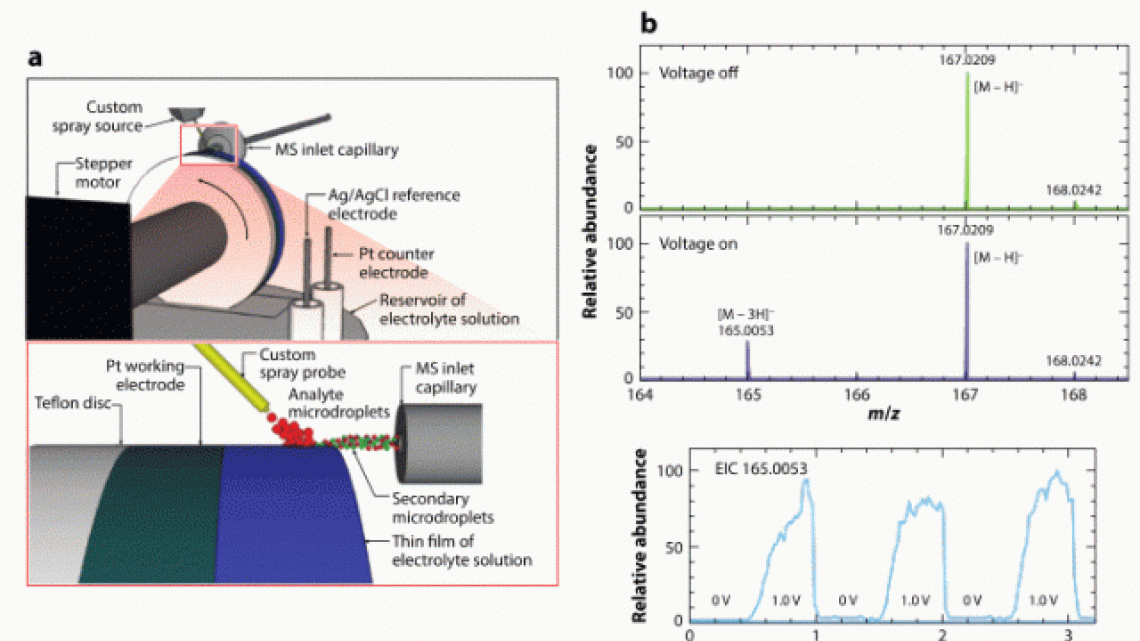
Scheme 2. Waterwheel setup allowing detection of transient electrochemical reaction intermediates from electrode surface by MS. Deprotonated diimine intermediate resulting from uric acid oxidaiton was detected at m/z 165, as shown in the right panel.
We have extended the desorption electrospray ionization (DESI),4 an ambient ionization method, to the direct analysis of liquid samples.
Due to its direct sampling and ionization properties, this liquid sample DESI-MS (LS-DESI-MS)5 is versatile and has unique applications.
For instance, it allows us to probe trasient electrochemical reaction intemediates6-8. Using a Waterwheel setup (Scheme 2), DESI-MS can be used to directly monitor electrochemical reactions from a rotating electrode surface.
Furthermore, we applied electrochemistry to assist the investigation of photoredox reaction mechanisms by MS.9
References
1. P. Zhao, R. N. Zare*, H. Chen*, J. Am. Soc. Mass Spectrom. 2019, 30, 2398-2407.
2. P. Zhao, Y. Guo*, H. D. Dewald*, H. Chen*, Int. J. Mass Spectrom. 2019, 443, 41-45.
3. C. Xu, Q. Zheng, P. Zhao, J. Paterson, H. Chen*, J. Am. Soc. Mass Spectrom. 2019, 30, 685–693.
4. Takats, Z.; Wiseman, J. M.; Gologan, B.; Cooks, R. G*. Science, 2004, 306, 471.
5. Miao, Z.; Chen, H*. J. Am. Soc. Mass Spectrom. 2009, 20, 10.
6. T. A. Brown, H. Chen*, R. N. Zare*, Angew. Chem. Int. Ed., 2015, 54, 11183 –11185
7. T. A. Brown, H. Chen*, R. N. Zare, J. Am. Chem. Soc. 2015, 137, 7274.
8. T. A. Brown, N. Hosseinei-Nassab, H. Chen*, R. N. Zare*, Chemical Science, 2016, 7, 329.
9. Y. Cai, J. Wang, Y. Zhang, Z. Li, D. Hu, N. Zheng*, H. Chen*, J. Am. Chem. Soc., 2017, 139, 12259.
10. Li, J.; Dewald, H. D.; Chen, H*. Anal. Chem., 2009, 81, 9716.
11. Y. Zhang, W. Cui, H. Zhang, H. D. Dewald, H. Chen*, Anal. Chem. 2012, 84, 3838.
12. Y. Zhang, H. D. Dewald, H. Chen*, J. Proteome Res. 2011, 10, 1293.
13. Q. Zheng, H. Zhang*, H. Chen*, Int. J. Mass Spectrom. 2013, 353, 84.
14. Q. Zheng, H. Zhang, S. Wu, H. Chen*, J. Am. Soc. Mass Spectrom. 2016, 27, 864-875.
15. Q. Zheng, H. Zhang*, L. Tong, S. Wu, H. Chen*, Anal. Chem. 2014, 86, 8983-8991
16. Q. Zheng, Y. Liu*, Q. Chen*, M. Hu, R. Helmy, E. C. Sherer, C. J. Welch, H. Chen*, J. Am. Chem. Soc., 2015, 137, 14035-14038
17. R. Cai, M. Lu, E. Aguilera, Y. Xi, N. Akhmedov, J. Petersen, H. Chen*, X. Shi*, Angew. Chem. Int. Ed., 2015, 54, 8772 –8776
18. H. Peng, R. Cai, C. Xu, H. Chen*, X. Shi*, Chemical Science, 2016, 7, 6190–6196.
19. J. Wang, S. Zhang, C. Xu, L. Wojtas, N. G. Akhmedov, H. Chen, X. Shi,* Angew. Chem. Int. Ed., 2018, 57, 6915 –6920.
20. M. Lu, Y. Su, P. Zhao, X. Ye, Y. Cai, X. Shi,* E. Masson,* F. Li, J. L. Campbell, H. Chen*, Chem. – Eur. J., 2018, 24, 2144-2150
21. X. Ye, P. Zhao, S. Zhang, Y. Zhang, Q. Wang, C. Shan, L. Wojtas, H. Guo*, H. Chen*, X. Shi,* Angew. Chem. Int. Ed., 2019, 58, 17226 –17230.
22. J. Wang, C. Wei, X. Li, P. Zhao, C. Shan, L. Wojtas, H. Chen, X. Shi*, Chem. Eur. J. 2020, 26, Accepted.
23. T. Yuan, P. Zhao, S. Teng, Y. Yi, J. Wang, C. Shan, L. Wojtas, J. Jean, H. Chen, X. Shi*, Chem, 2020, Accepted.
24. H. Chen, L. S. Eberlin, R. G. Cooks*, J. Am. Chem. Soc. 2007, 129, 5880.
25. H. Chen, L. S. Eberlin, M. Nefliu, R. Augusti, R. G. Cooks*, Angew. Chem. Int. Ed., 2008, 47, 3422.
26. P. Liu, P. Zhao, R. G. Cooks*, H. Chen*, Chemical Science, 2017, 8, 6499.
27. P. Zhao, T. White, R. G. Cooks,* Q. Chen,* Y. Liu, H. Chen*, J. Am. Soc. Mass Spectrom. 2018, 29, 2317–2326.
28. Y. Cai, D. Adams, H. Chen*, J. Am. Soc. Mass Spectrom. 2014, 25, 286.
29. Y. Cai, Y. Liu, R. Hemly, H. Chen*, J. Am. Soc. Mass Spectrom. 2014, 25, 1820.
30. Y. Cai, Q. Zheng, Y. Liu, R. Helmy, J. A. Loo, H. Chen*, Eur. J. Mass Spectrom. 2015, 21, 341–351.
Hao Chen
hao.chen.2@njit.edu