


Located in the Department of Chemistry & Environmental Science at the New Jersey Institute of Technology(NJIT), headed by Professor Somenath Mitra.Our research focus are in the field of Analytical Chemistry, Nanotechnology and Smart Active Coatings. In analytical chemistry we are geared towards developing instrumentation for on-line/ real-time monitoring analysis, environmental monitoring, field portable instruments and micro fluidic devices. In nanotechnology we work on nanoparticles, particularly Carbon Nanotubes (CNTs) as an adsorbents for various environmental/pharmaceutical pollutants, chromatography stationary phases, expanding their applications by functionalization and polymer composites. Third area of our group's focus is on activity Smart Active Coatings with embedded sensing and color change properties with potential application in defense related objects funded by Department of Defense (DOD).
Published Books




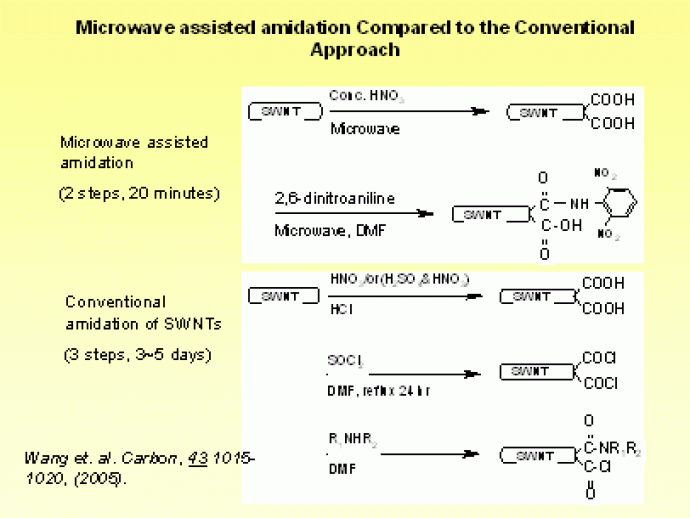





 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Haloacetic acids are an important class of disinfection byproducts that are being regulated. We have developed novel instrumentation for continuous monitoring of the nine haloacetic acids. Hollow fiber liquid-liquid membrane extraction (LLME) and supported liquid membrane extraction (SLME) followed by on-line HPLC-UV detection were studied. With continuous LLME, seven halo-acetic acids could be analyzed and enrichment factor (EF) was around 50. All the nine acids could be extracted and quantified by continuous SLME. Experiments with laboratory standards demonstrated that EF and extraction efficiency could be as high as 500 and 54% respectively. Relative standard deviations based on seven replicates were between 3.3 and 10.3 %, and the MDLs were at sub-ppb levels.

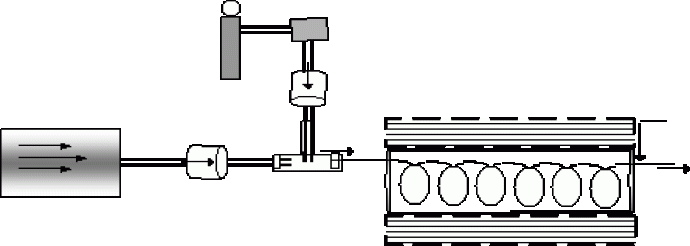
Figure 1: Schematic diagram of the system used for the continuous monitoring of haloacetic acids in water.

Figure 2. Chromatogram of continuous SLME of reagent water spiked with 80 ng/mL (ppb) nine HAAs. The donor flow rate was 4 ml/min. The acceptor was 0.05 M tris buffer (PH 8.7) with a flow rate of 0.005 ml/min. Injections were made every 15 min. The numbered peaks in the chromatogram are: 1: MCAA; 2: DCAA; 3: MBAA; 4: BCAA; 5: DBAA; 6: TCAA; 7: BDCAA; 8: CDBAA; and 9: TBAA.

Fig. 3 Microfluidic chip made of polycarbonate material into which microchannels have been machined.
References
- “Continuous on-line monitoring of haloacetic acids via membrane extraction”. X. Wang and Somenath Mitra. J. of . Chromatogr. A. 1089, 39-44 (2005).
- “Microfluidic supported liquid membrane extraction”. X. Wang and S. Mitra. Analytica. Chimica. Acta. 543, 92-98, (2005).
Fast and Simple Monitoring of Haloacetic Acids in Water by Supported Liquid Membrane Micro-Extraction (SLMME) with HPLC Detection
Prior to the development of a continuous monitoring system, supported liquid membrane microextraction (SLMME) had been developed for the extraction, preconcentration, and determination of all the nine haloacetic acids (HAAs) in water. The HAAs are extracted into a supported liquid membrane, and then back-extracted into few microliters of an acceptor solution. Enrichment factors, as high as 4000, and low to sub-ppb detection limits were obtained in a 60 minute extraction. The extract was directly analyzed within ten minutes by flow programmed HPLC-UV. The detection limits were at ng/L (ppt) levels, and RSDs were between 2 and 12%. The parameters that affected analyte enrichment were studied. This approach offers an attractive alternative to the current standard EPA methods for HAAs, which require complex sample preparation and derivatization prior to GC analysis. SLMME can be used in conjunction with other detection schemes, such as, ion chromatography and capillary electrophoresis.
References
- “Development of supported liquid membrane microextraction for the trace determination of haloacetic acids”. D. Kou and S. Mitra, J. of Chromatogr. A, 1055, 63-69 (2004).
- "Microfluidic supported liquid membrane extraction”. X. Wang and S. Mitra. Analytica. Chimica. Acta. 543, 92-98, (2005).
Membranes have become a viable alternative to conventional sample preparation techniques such as liquid-liquid extraction (LLE), solid-phase extraction (SPE), rotary evaporation and solid phase microextraction (SPME). They have been applied to a wide range of compounds. Below are a few examples of the versatility of membrane extraction techniques.
Continuous On-line Monitoring of Haloacetic Acids
Disinfections by-products (DBPs) refer to those compounds formed upon the addition of disinfecting agents such as chlorine, chlorine dioxide and chloramines for the purpose of water treatment. One such group of DBPs are haloacetic acids(HAAs). Many of these compounds have been determined to be hazardous to human health and as such are monitored by the EPA. The EPA methods (EPA method 552.1, 552.2 and 6251)methods involve cumbersome liquid–liquid extraction or ion exchange and derivatization, followed by GC-ECD detection and therefore is time consuming, consume large amounts of solvent and not amenable to automation. We have recently developed an automated on-line system capable of continuous real- time monitoring as well as a microfluidic system.
An automated on-line system allows us to get real-time data and reduces sample handling and therefore is less prone to errors. Microfluidic devices shown in Fig. 1 have large surface area to volume ratios, consume small amount of reagents and can be inexpensively produced. With these systems, the detection of all nine HAAs at ppb levels was possible. Both techniques are based on supported liquid membrane extraction (SLME) which is essentially a three-phased system where an organic phase is sandwiched by two aqueous phases. The analyte (A-) moves across a pH gradient and are trapped in the acceptor phase as shown below.
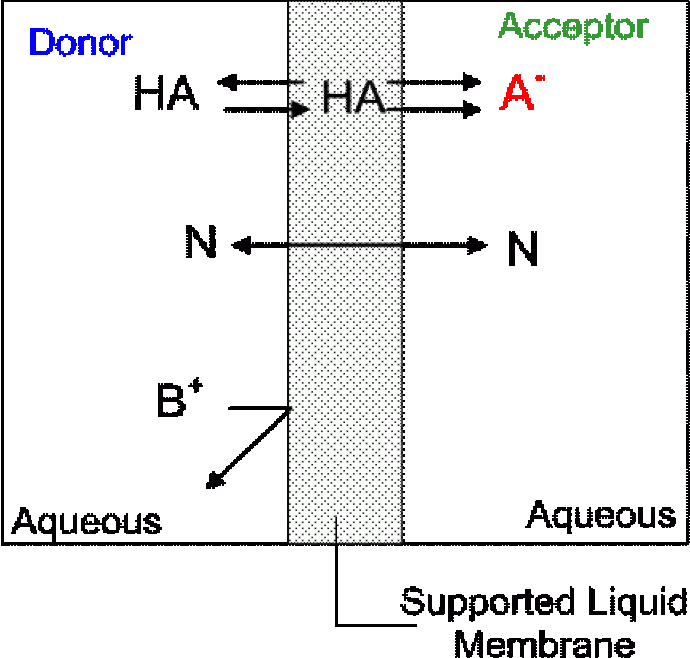
Fig. 1 Schemmatic representation of SLME of acids
Barrier-film Microscale Membrane Extraction
Membrane extraction typically involves the use of an organic liquid immobilized within the pores of a hydrophobic porous membrane. The extraction is dependent upon this liquid remaining in the membrane pores throughout the procedure. This liquid may be lost via diffusion or dissolution in the acceptor solution. Loss of the extractant results in analyte loss and consequently low enrichment factors (EF).
To enhance membrane stability we have used a dip-coating procedure to coat the membrane in a liquid immiscible with the acceptor. This liquid is referred to as the barrier film. This is shown below. This facilitates more rigorous extractions, decreases extractant loss and results in higher EFs. This technique has been successfully used to detect polyaromatic hydrocarbons such as anthracene, fluorene and phenanthrene in water at parts per trillion levels as well as carbamate pesticides such as carbaryl, carbofuran and methiocarb.
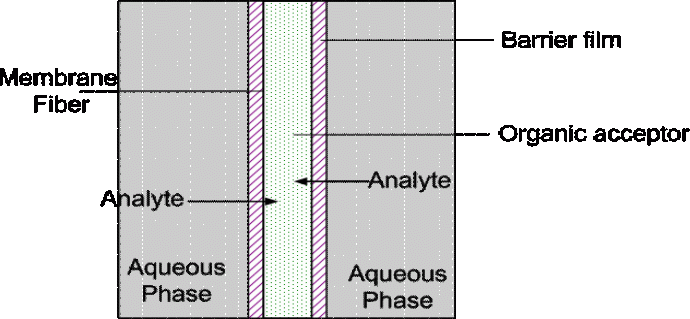
Fig. 2 Schemmatic diagram of barrier-film enhanced SLME. The barrier film coats the membrane and so stabilizes the organic extractant and facilitates greater enrichment of the analytes in the membrane lumen.
Pervaporative Concentration of Pharmaceuticals
Whereas the other techniques were geared towards semi-volatile organic compounds (SVOCs), pervaporation is suitable for volatile organics and in this case refers to the permeation of volatile organics across a membrane barrier into a gas phase. Continuous on-line analysis has been essentially non-existent in pharmaceutical manufacturing. Typically, samples are collected at various steps in the process, and sent to the laboratory for analysis.
The samples undergo various sample preparation steps, such as, extraction and concentration prior to detection. These steps are both labor and time intensive. Evaporative techniques are usually used for analyte concentration. Essentially, it concentrates the sample by selectively removing the solvent. Most classical evaporative techniques are relatively laborious procedures involving multiple handling steps which tend to results in increased error. We have been able to develop on-line membrane preconcentration for monitoring pharmaceuticals at trace levels. Using polar solvent permeable Nafion membranes, solvent was reduced by more than 90% allowing the concentration of compounds such as 1,2-diphenylhydrazine and naphthylacetonitrile shown in Fig. 3
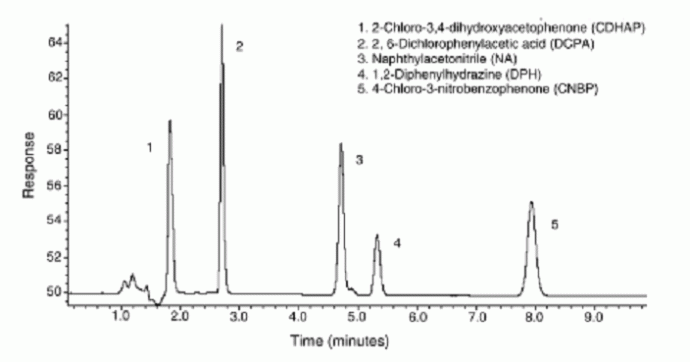
Fig. 3 HPLC separation for 5 pharmaceuticals after membrane pervaporation
High performance stationary phases, which provide high resolutions and are stable at high temperatures, are of significant importance in gas chromatographic analysis. Carbon nanotubes are nano-sized carbon-based sorbents, which have high surface area, large aspect ratio and are known to be stable at high temperatures. Therefore gas chromatography can benefit from their unique properties. Gas chromatography separations in an open tubular format on self-assembled single walled carbon nanotubes (SWNTs) is being performed with an average thickness of 300 nm, which is self-assembled by a unique single-step, catalytic chemical vapor deposition (CVD) process consisting of dissolved cobalt and molybdenum salts in ethanol.
A variety of organic compounds with varying polarity can separated at high resolution and the column efficiency demonstrated around 1000 theoretical plates/m. The range of compounds that can be separated by GC on sa single column ranges from small molecules like methane to large molecules like PAHs. Figure 1 shows a separation range from C1 to C14 hydrocarbons. This extremely wide range is attributed to high capacity adsorption followed by relatively easy and fast desorption from the high aspect ratio CNTs. This is truly a nano-effect of these ultra small sorbents.
Comparison of capacity factors (k’) and isosteric heats of adsorption (DHs) with a packed column containing a commercial sorbent (Carbopack CTM) showed comparable results. This demonstrated high capacity and strong sorbate-sorbent interactions on the SWNT phase. Evaluation of the McReynolds constants suggested that the SWNT was a non-polar phase.
References
- “Chromatography of self-assembled singe walled carbon nanotubes”. M. Karwa and S. Mitra, Anal. Chem. 78, 2064 (2006).
- "Chromatography on Self-Assembled Carbon Nanotubes.”. Chutarat Saridara and Somenath Mitra.77(21), 2094 Anal. Chem. (2005).
- “Selective Self-assembly of Single Walled Carbon Nanotubes in Long Steel Tubing for Chemical Separation”. Mahesh Karwa, Zafar Iqbal and Somenath Mitra. J. of Mater. Chem. 16, 2890 - 2895 (2006).
For any queries, inquiries or clarifications please contact:
Professor Somenath Mitra
(973) 596 - 5611
(973) 596 - 8436 (fax)
mitra@njit.edu
Mailing Address
321 Y Building.
New Jersey Institute of Technology
138 Warren Street
Newark, NJ 07103